CIHR Internal Assessment - Report for the 2011 International Review
Part 4: CIHR 2005–2010
Achievements related to Blueprint, CIHR's first strategic plan
The first five-year strategic plan, CIHR's Blueprint for Health Research and Innovation, or Blueprint, was published in 2003–2004. Therefore, during the first International Review in 2006, CIHR was in the middle of the period covered by Blueprint. Now that CIHR's second strategic plan, Health Research Roadmap: Creating innovative research for better health and health care, 2009–2010 to 2013–2014, or Roadmap, has superseded it, CIHR's can report on its Blueprint objectives.
Box 1: Blueprint's five strategic directions
- Strengthen Canada's health research communities.
- Address emerging health challenges and develop national research platforms and initiatives.
- Develop and support a balanced research agenda that includes research on disease mechanisms, disease prevention and cure, and health promotion.
- Harness research to improve the health status of vulnerable populations.
- Support health innovations that contribute to a more
Blueprint committed CIHR to follow five strategic directions (Box 1). Highlights of CIHR's actions that address the five strategic directions, and in which the institutes have been key players, include:
-
support for new funding programs to encourage high-risk research and novel collaborations, with the growth of CIHR-funded researchers from 9,640 in 2003–2004 to 13,790 in 2009–2010
-
rapid launching of research programs that address emerging threats to public health including SARS and pandemic influenza
-
support for large cohort studies, research data centres and the Cochrane Collaboration; launch of several joint institute/corporate-funded large strategic initiatives, e.g., Regenerative Medicine and Nanomedicine (Box 2), and Global Health
-
continued growth in the support for research in health services and population health research (Figure 4B)
-
an emphasis on the health of vulnerable populations, with a quadrupling of funding in this area from $10.8 million to $44.6 million between 2003–2004 and 2008–2009
-
a revitalized approach to KT, emphasizing partnerships with knowledge users and researcher education about the relevance and meaning of KT to all themes of health research, to the extent that the KT function is one of the features of CIHR that is best known internationally
Box 2: CIHR Regenerative Medicine and Nanomedicine Initiative
The Regenerative Medicine and Nanomedicine Initiative (RMNI) is one of CIHR's most successful large strategic initiatives, having committed $84 million on Team and Catalyst grants since 2003. Although the RMNI topic does not fall clearly within the mandate of any one Institute, CIHR recognized its importance and the Institute of Genetics, the Institute of Musculoskeletal Health and Arthritis and the Institute of Neurosciences, Mental Health and Addiction spearheaded the development of the initiative, which now involves eight Institutes, the Ethics Office and the KT Portfolio, as well as 12 partner organizations, including the Canadian Space Agency.
Annual funding opportunities are available in nanotechnology applied to health, stem cells, tissue engineering, rehabilitation sciences and related ethical, economic, environmental, legal and social issues. RMNI has also held a large number of workshops, including a Canadian Workshop on Multidisciplinary Research on Nanotechnology in 2008. Organized by the Tri-Council, along with Health Canada, the National Research Council, Environment Canada and Industry Canada, the workshop brought together researchers and representatives from government, industry and citizens' groups. The participants identified emerging issues in nanotechnology, including its ethical and economic implications, potential impact on the environment and public health and gaps in regulation and policy.
CIHR's investment in RMNI seems to be paying off in terms of high-quality Canadian research. In both regenerative medicine and nanomedicine, Canada's share of world publications has increased, as has their citation impact.
CIHR expected that these actions would result in a number of outcomes, grouped under five broad headings. Table 6 lists in detail the broad and specific Blueprint objectives, and in the second column are listed the actions to which CIHR committed. The third column reports on the outcomes achieved; in some cases, these are described in quantitative terms, in others as examples of actions taken. Many of the individual items mentioned in the table are described in more detail elsewhere in this report and in the institute reports.
Table 6: Blueprint achievements
Outstanding research
| Objective | Action | Achievement |
|---|---|---|
| 1. Support the creative proposals of excellent Canadian health researchers across the full spectrum of health research. | Strengthen Canada's capacity for excellent and ethical health research by providing grants, adequate in number and value, to support the very best proposals of individuals and teams of health researchers. | Increased number and value of grants and introduced Catalyst, New Team and Team Grants to encourage high-risk research and the support of new and established collaboration. |
| Support applications from all research communities relevant to health, to continue to broaden the scope of CIHR-funded research. | Funded more researchers (9,593 in 2003–2004, 13,695 in 2009–2010) and institutions, especially in health services and population health themes. | |
| 2. Stimulate and sustain research that capitalizes on key scientific opportunities, addresses important and emerging health issues of concern to Canadians and contributes to the health of society worldwide. | Support excellent, ethical and innovative research responsive to Institute-identified research priorities. | Introduced large number of institute-sponsored research programs, many described in institute reports. |
| Increase support for research that contributes to improvements in the health status of vulnerable populations. | Increased funding for research related to vulnerable populations from $10.8 million in 2003–2004 to $44.6 million in 2009–2010. | |
| Increase support for research initiatives in health promotion and disease prevention. | Increased funding for research related to prevention and health promotion from $19.8 million in 2003–2004 to $55.5 million in 2009–2010. | |
| Respond to emerging health threats with targeted programs of health research support. | Rapid response to SARS, pandemic influenza, isotope shortages. | |
| 3. Encourage and support interdisciplinary, collaborative research designed to resolve complex health issues. | Establish and sustain innovative programs for interdisciplinary collaborative research that are accessible to investigators working in all areas of health research. | Introduced Team and New team grants. Funding for interdisciplinary programs increased from $81.1 million in 2003–2004 to $112.5 million in 2009–2010. |
| Enhance the ability of CIHR's peer review system to appreciate and evaluate interdisciplinary collaborative research proposals. | Created new interdisciplinary peer review panels, e.g. Gender and Health, Children's Health. | |
| Support research on the ethical, legal and sociocultural issues related to health and the delivery of health care as an integral part of the multidisciplinary approach to complex health problems. | Created special funding allocation for ethics research, created new peer review committee. Ethical issues a required part of training programs. | |
| 4. Stimulate research activities that accelerate the translation of health research into action. | Strengthen support for intervention research and clinical trials with potential to directly affect quality of care, quality of life and the effectiveness of the health system. | Increased funding for clinical trials, support for larger and multinational clinical trials. Clinical trials can now also be funded from open operating grants budget allocation. |
| Establish and sustain innovative programs to support researcher collaboration with the industry sector. | Established Centres of Excellence for Commercialization of Research. Enhanced Proof of Principle program. | |
| 5. Increase Canadian contribution and visibility in international initiatives in health research | Support selected, large-scale international initiatives where Canadian researchers lead or make a unique contribution to international efforts. | Structural Genomics Consortium, Cancer Stem Cell Consortium, Grand Challenges in Global Health and HIV/AIDS Vaccines |
| Increase the number of bi- or multilateral collaborative agreements with research agencies in other nations, in priority areas. | China-Canada Joint Health Research Initiatives, Canada-China Norman Bethune Health Research Scholarships Program, India-Canada Collaborative Teams in Childhood Obesity Research, Canada-Finland Team Grant: Early origins of addiction in children and youth | |
| Improve opportunities for Canadian researchers to participate in research activities funded by international agencies, including providing support for establishing collaboration and developing proposals. | Made changes to Grants and Awards Guide to publicize and facilitate opportunities for international collaboration through CIHR's major funding programs. Created and expanded the Global Health Research Initiative. |
Outstanding researchers in innovative environments
| Objective | Action | Achievement |
|---|---|---|
| 1. Build health researcher capacity across the broad spectrum of health research in a vibrant, innovative and stable research environment. | Increase the supply of health researchers in areas of need identified by institutes by supporting early and mid-career training opportunities. | Increased support for New Investigators, Canada Research Chairs, Tier 2. Institute-sponsored career awards increased from $4.7 million and 115 awards in 2003–2004 to $11.6 million and 191 awards in 2009–2010. |
| Increase the number of outstanding new investigators and retain established researchers, with special attention to increasing the participation of women and Aboriginal people in health research. | Number of investigators supported with salary awards increased from 1,081 in 2003–2004 to 1,222 in 2009–2010. Number of investigators funded for IAPH-relevant research increased from 350 in 2003–2004 to 574 in 2009–2010. IAPH established Network Environments for Aboriginal Health Research to provide an appropriate environment and resources that would encourage Aboriginal and non-Aboriginal students to pursue careers in Aboriginal health research. | |
| Provide programs designed to attract and repatriate outstanding health researchers to Canada from abroad. | Tri-Council Canada Research Chairs, Chairs of Excellence, Vanier Scholarships, Banting Fellowships | |
| Complement and build on current research capacity building initiatives and programs established by relevant stakeholders (e.g. Canada Research Chairs, CFI, Canada Graduate Scholarships). | Established CIHR-NSERC joint Collaborative Health Projects, which supports projects involving any field of the natural sciences or engineering and the health sciences; CFI and CIHR joint proposals for regional/national clinical research initiatives, combining infrastructure and operating support. | |
| Support programs and networks designed to reduce regional disparities in the training and establishment of researchers. | Evaluated Regional Partnerships Program in 2005 and showed it was successful. Renewed the program with some changes to make it more responsive to provincial priorities. | |
| Support policies, systems and practices that promote a culture of ethics and integrity in health research. | Introduced compulsory registration and disclosure of outcomes of CIHR-funded clinical trials. Introduced open-access policy. | |
| 2. Develop, support and sustain new national platforms and initiatives for health researchers. | Build the Canadian Lifelong Health Initiative with partners: a major longitudinal and intergenerational study to follow cohorts of newborns and seniors to delineate the genetic, psychosocial, cultural, economic and environmental determinants of health and healthy aging. | Embarked on three large cohort studies: CHILD Study, Canadian Longitudinal Study on Aging and the Canadian Partnership for Tomorrow Project (cancer and chronic diseases: supported by Canadian Partnership Against Cancer and regional cancer agencies). |
| Establish a modernized Canadian platform for clinical research including national networks, core facilities, sustainable support mechanisms for clinician researchers, and innovative mentoring and training opportunities. | Engaged in significant consultations with clinical research community, resulting in CIHR's Strategy on Patient-Oriented Research (SPOR). Invested with CFI in improving clinical research infrastructure in Canada. | |
| Support initiatives intended to develop, expand and refine research approaches and methods used by researchers. | Launched several programs focused on development of tools and methodologies, e.g., Catalyst Grants for Invention – Tools, Techniques and Devices to encourage Canadian investigators to develop novel tools and techniques, or novel applications of existing tools and techniques; Catalyst grants in Population and Public Health for development and validation of new inventions, tools, methodologies, protocols, theoretical models or frameworks. | |
| Support the development of, and improve access to, health and health services data to enable researchers to undertake outstanding research. | Supported Statistics Canada research Data centres at Canadian Universities. | |
| 3. Engage Canadian youth in health research. | Develop and implement programs to initiate young Canadians into health research. | Developed and implemented Youth Engagement Strategy. Formed partnerships with existing groups similarly engaged. |
| 4. Enhance and sustain supportive research training environments and networks. | Support innovative training programs that provide students and post doctoral fellows with experience in interdisciplinary, collaborative and inter-sectoral (e.g. industry, policy, community-based) research environments. | Funded second round of 50 training programs in 2008, representing an $89 million commitment over six years. In collaboration with other federal agencies, support Industrial R&D Internship Program, which provides opportunities for graduate students and PDFs to undertake short-term research projects in collaboration with an industrial partner. |
| Support mentorship of new researchers and establish networks of collaboration and support. | Many institutes have developed trainee and new investigator workshops: networks develop as a result of many New Team and Team Grant projects. |
Transforming research into action
| Objective | Action | Achievement |
|---|---|---|
| 1. Advance research in the use of health knowledge. | Support research that seeks to determine the most effective strategies and techniques for dissemination and exchange of knowledge created through health research. | Projects on the science of KT funded as Open Operating Grants, reviewed by new KT peer review committee. Additional grants funded through Priority Announcements and KT portfolio. $20.2 million invested since 2004–2005. |
| Support research designed to determine effectiveness of new or changed health policies, programs and practices. | Investments in health services and policy research increased from $32 million in 2003–2004 to $55.5 million in 2009–2010. | |
| Work in partnership with research institutions, other government agencies and industry to ensure timely commercialization of intellectual property derived from research. | Introduced Proof of Principle program, subsequently tuned to provide better assistance. Introduced Science to Business (S2B) program designed to encourage individuals with PhDs in a health related field to pursue an MBA. | |
| 2. Develop and sustain a broad range of individuals involved in the exchange and use of health knowledge. | Provide programs designed to support a culture change among health researchers and institutions by supporting students, postdoctoral fellows, young investigators, and scholars and their mentors who demonstrate a commitment not only to conducting outstanding health research, but also to working collaboratively with potential users of research in ways likely to improve KT. | Introduced end-of-grant funding supplements to assist KT of research findings. Introduced prestigious CIHR Knowledge Translation Award. Developed KT Capacity Development Initiative supporting KT trainees. Published a KT Handbook for researchers. Developed educational modules, Summer Institutes for researchers and trainees and the KT Trainee network. Developed and implemented Policy on Access to Research Outputs. Established Commercialization Advisory Committee. |
| 3. Develop and sustain innovative environments that enable the effective use of health knowledge. | Develop and support programs in partnership with stakeholders that bring together partners and bridge the gaps between research, practice, programs and policy. | Established Partnerships for Health System Improvement (PHSI) program. PHSI supports teams of researchers and decision makers conducting applied and policy-relevant health systems and services research that responds to the needs of health care decision makers. Developed Knowledge to Action program to build KT capacity at community, regional or provincial level. |
| Develop and implement mechanisms that foster effective communications and enable researchers and users of research knowledge to build productive relationships. | Introduced Best Brains Exchanges and Expedited Knowledge Synthesis, which provide rapid expert advice in response to specific needs of provincial health policy makers. Provided grants for planning and dissemination for PHSI grant teams of knowledge producers and users. Synthesis grants support teams of researchers and knowledge users to produce syntheses and scoping reviews of importance to knowledge users. | |
| Support initiatives that will identify effective approaches for users to translate health knowledge. | Publicized success stories from all CIHR-supported initiatives through four KT Casebooks. | |
| Monitor and evaluate worldwide approaches to knowledge translation, and engage with international partners who have a similar mandate or interest. | An ongoing activity of the Knowledge Synthesis and Exchange Branch. CIHR's policies in the area of trial registration have contributed to international standards, and its policy on open access has become the de facto Canadian standard for other funders. CIHR's KT approaches have attracted favorable international comment. |
Effective partnerships and public engagement
| Objective | Action | Achievement |
|---|---|---|
| 1. Engage in mutually beneficial international partnerships. | In consultation with stakeholders, develop and implement a comprehensive framework to guide partnership activities. | Published The CIHR Partnerships Casebook to advertise examples of a variety of successful partnerships. |
| Promote productive relations with relevant international stakeholders to gain synergies, enlarge scope of inquiry and pool resources. | Numerous international partnerships created: from 14 in 2003–2004 to 32 in 2009–2010. | |
| Develop and implement initiatives and programs that promote international and best practices, excellence and ethics in health research. | Partnered with UK and Australia in Project Retrosight to identify payback from health research. PubMed Central Canada launched in 2010. | |
| 2. Develop and maintain a broad base of stakeholder support across Canada. | Establish and maintain collaborative relationships with stakeholders to meet organizational mandate and goals. | Established Leaders Forum where all sectors of research funders meet regularly. Established numerous Institute-sponsored workshops and consensus processes for setting research agendas in targeted areas. |
| Develop and implement proactive strategies to attract and secure partnership opportunities. | Developed Partnership Management Strategy to assist CIHR staff in forming and maintaining productive partnerships. | |
| 3. Develop and maintain a coherent and coordinated approach to research across the full spectrum of health research. | Pursue and secure partnerships with organizations in Canada that share common values and goals in the area of health research. | Formed multiple partnerships: from 265 agreements in 2003–2004 to 382 in 2009–2010. |
| 4. Enhance public and stakeholder engagement in health research in Canada. | Engage in ongoing dialogue with the Canadian public and other stakeholders to heighten awareness of the significant role health research plays in improving the health of Canadians, the health system and the effectiveness of products and services. | Developed Public Engagement Strategy, including Café Scientifiques program across Canada. |
| Involve the Canadian public and other stakeholders in priority-setting and appropriate research activities (e.g. peer review panels, forums of various institutes). | Added community members to many review panels, including citizen representation on Institute Advisory Boards. Increased use of merit review where both research peers and knowledge users evaluate applications. | |
| 5. Promote science to Canadian children and youth. | Create opportunities in collaboration with partners to engage children in science discovery (e.g. GEE in GENOME travelling exhibit, Discovery Days). | Launched Synapse youth outreach program. |
Organizational excellence
| Objective | Action | Achievement |
|---|---|---|
| 1. Provide leadership and coordination in setting direction on important health research issues. | Ensure that CIHR's research agenda remains current through ongoing consultations with a broad range of stakeholders. | Established Leaders Forum where all sectors of research funders meet regularly. Established numerous institute-sponsored workshops and consensus processes for setting research agendas in targeted areas. |
| Contribute to the development of innovative public policies related to ethical, legal and socio-cultural issues in health and health research. | CIHR Guidelines for Human Pluripotent Stem Cell Research 2002, updated 2007, and CIHR Stem Cell Oversight Committee 2003 provide framework and national oversight of human stem cell research. Completed work on policies for health research involving Aboriginal people and research misconduct. Initiated policies for research involving children and for relationships with the private sector. | |
| 2. Promote CIHR's research agenda and ensure that the needs of the scientific communities are effectively met. | Develop and implement processes designed to respond effectively to the needs of research communities representing the full spectrum of research. | Amended peer review process to eliminate possible disadvantage to non-biomedical applications following research on rating patterns of review committees. |
| Promote institute research priorities at all levels of research, policy and practice in Canada and abroad. | Created Scientific Council, which allows direct input of institute priorities to CIHR decision making. | |
| 3. Build a committed, motivated and productive workforce across the organization. | Develop and implement a continuous learning environment within CIHR for all staff. | Required all staff to develop learning plans in collaboration with their managers. |
| Develop and implement new job classification, evaluation and compensation systems that recognize performance. | Implemented all components. | |
| Develop and support a healthy work environment. | CIHR recognized as one of Canada's 100 top employers for 2010. | |
| 4. Improve overall organizational effectiveness through ongoing improvements in programs, structures and processes. | Develop and implement governance renewal processes and mechanisms to support excellence in governance. | Implemented routine mechanisms for institute director renewal and transition. |
| Advance an institute-centred organization through effective alignment of programs, structures and processes with institute priorities and requirements. | Created Scientific Council, which places institutes at centre of CIHR decision making. The rationalization of competition timetable and complexity of funding opportunities increases visibility of strategic priorities. | |
| Develop and implement a framework that enables the evaluation of the organization's performance and the value of its programs of research support. | Substantially increased evaluation and analysis capacity and competence, with significant studies completed. | |
| Enhance the effectiveness of CIHR's peer review system. | Reduced time and cost factors in several areas, e.g. 'at home' reviews for training awards. Introduced triage to eliminate uncompetitive applications. | |
| Develop and implement a risk management framework and mitigate priority risks. | Implemented risk management in accordance with federal directives. | |
| Advance modern management practices to ensure that CIHR meets Government of Canada objectives set out in Results for Canadians: A Management Framework for the Government of Canada. | Achieved full compliance with federal requirements for accountability and reporting results. | |
| 5. Capitalize on technology to enhance service delivery. | Continue to promote and support the implementation of electronic service delivery such as the Common CV project. | CommonCV now used by 17 Canadian research agencies, including NSERC, SSHRC, Genome Canada, CFI. |
| Support the roll-out of ResearchNet, a Canadian research portal that supports collaboration and information sharing among researchers, research organizations, government, industry and the public. | ResearchNet now used in major CIHR competitions. | |
| Leverage technology to improve the efficiency and effectiveness of business processes (e.g. peer review, website applications such as e-applications for funding). | Electronic submission of applications used in major competitions, including open operating grants. | |
| Enhance databases and information, including public access to information about CIHR's investments in research. | Refined CIHR's fully searchable public funding database. |
Results of and responses to first International Review
CIHR's first International Review Panel (IRP) concluded that CIHR was meeting its mandate and all 13 institutes were working well. The IRP was impressed by the progress made in developing a more unified model of health research funding. The capacity to fund research across all health-related disciplines had clearly been enhanced and new strategic initiatives had strengthened multidisciplinary research and training. The IRP concluded that it was too early to make conclusive judgments as to the effectiveness of the CIHR model of health research funding. The report offered observations for CIHR to consider in the next stage of its evolution. These are listed below in italics, with CIHR's response. In some cases, the IRP's advice validated actions already in progress.
Governance and management
"Accountability and transparency need to be reinforced at all levels of the organization. Governing Council (GC) should consider its position as the main board of the organization and a single research committee should be established to account for all research expenditures."
GC has clarified its strategic governance role and assumed the role of Board of Directors. It has delegated its responsibilities for scientific matters, including approval of funding awarded in CIHR's competitions, to a newly constituted Scientific Council. GC now concentrates on broad strategic direction setting and provides strategic advice.
"Rapid growth and the challenges associated with matrix management across the CIHR Institutes and Ottawa has created management challenges within CIHR leading to the conclusion that the executive team needs expanding and strengthening. The most appropriate structure for handling these issues should be considered after an organizational review."
Two organizational reviews were conducted by external consultants and their recommendations have been implemented to strengthen the management team, with realigned responsibilities (Table 1).
SDs play an expanded leadership role through their membership on the Scientific Council, which is responsible for the expenditure of the budget approved by GC. SDs are also responsible for overseeing performance of the entire peer review system, though not for its actual operation, which is the responsibility of the Research Portfolio."Scientific Directors (SD) should now be given further responsibility to oversee the panel/activity in their scientific area. It would also seem reasonable that a future role of SDs might be to form the core of the central committee replacing RPPC responsible for allocation of the whole research budget."
"The crucial leadership role played by the SDs led the IRP to consider the succession challenges associated with moving CIHR Institutes every five to seven years and believe this is a significant issue as institutional memory will be lost. Options should be considered for ensuring the smooth transition of the institutes."
Turnover of SDs is now routine, and dedicated resources and processes facilitate the transition. The increased experience of Ottawa-based institute staff ensures continuity of institutional memory. The benefits of regular institute leadership renewal outweigh the inevitable disruptions.
Programs and peer review
"Rapid growth, particularly of new strategic initiatives and peer review panels, has led to excessive complexity. This complexity needs to be reduced to enable opportunities and activities to be both focused and manageable."
The proliferation of funding programs has been costly with respect to peer review. For the 149 operating grant programs in operation in 2009–2010, review of applications involved 125 review committees. The Scientific Council has moved to reduce the number of strategic initiatives and focus on fewer, larger, multi-institute or pan-CIHR opportunities. It has adopted a critical, centralized approval system for strategic initiatives based on the number of institutes involved, scope, cost and duration.
"The peer review system that is responsible for handling most of the research funding is currently under strain and requires more academic leadership. A review of its processes and structure is necessary."
Reform of the peer review system is one of the key deliverables of CIHR's new strategic plan (Roadmap). Meanwhile, a streamlining process allows triage of uncompetitive the applications, enabling review committees to focus on those in contention.
"Since teams and collaborations often form unpredictably and in a more bottom-up approach in response to complex problems, CIHR should develop a flexible and responsive approach to promote multidisciplinary research."
The largest and most flexible program is the Open Operating Grant Program, which has no limitations on number of applicants or requested grant size and, increasingly, "self-assembling" research teams are applying for funding through these competitions.
Knowledge translation
"There remains lack of clarity about the definition of knowledge translation (KT) across the organization."
CIHR is now recognized as a leader among funding agencies in its approach to KT. "CIHR has taken a bold stand in leading the advancement of this agenda. Hopefully other funding agencies will follow suit".36 It has refined its definition of KT, making a distinction between "end of grant" KT, where information is disseminated to those who need to use it at the end of a research project, and "integrated" KT where the users of research knowledge are engaged in all phases of a research project, from formulating the research question to applying the findings. It has adopted the Knowledge to Action framework to guide its approach to KT.37
To educate the research community about the concepts and practical processes of KT, CIHR commissioned the development of four KT learning modules,38 produced a series of KT Casebooks,39 and published the book, Knowledge Translation in Health Care, which provides the state of the theory and research evidence behind studying and doing KT.40 CIHR also provides the majority of support for the work of the Cochrane Collaboration in Canada. Annual expenditures on grants and awards to support KT rose from $350,000 in 2001–2002 to $18.2 million in 2009–2010.
An important recent KT development is CIHR's Evidence on Tap program, designed to engage federal, provincial and territorial ministries of health by meeting their need to inform policy making with research evidence. This program brings together in Best Brains Exchanges CIHR-funded researchers and policy makers to discuss in confidence the evidence about the particular health issues on which a ministry is seeking advice. At the request of ministries, CIHR also funds research teams to provide expedited knowledge synthesis of the evidence in areas of interest. The Evidence on Tap program has been warmly received by policy makers: "Now you hear the Deputy Minister talk about CIHR all the time. He talks about Best Brains and says that what we're trying to do is accomplish fundamental change and that CIHR, with the Best Brains, is helping us to achieve this."41
Since CIHR's Institutes are strongly linked to the researcher and knowledge user communities, they play a critical role in promoting and facilitating the dissemination and application of CIHR research results. Increasingly, the institutes have embraced their role in supporting and promoting researchers' KT efforts as well as evolving their own role as knowledge brokers. To guide their individual approaches, institutes now include KT activities as integral parts of their strategic plans.
"More attention should be directed at providing leadership in the area of technology commercialization."
The introduction of the Tri-Council Centres for Excellence in Commercialization of Research and the business-led Networks of Centres of Excellence have boosted CIHR's spend on commercialization activity. Nevertheless, challenges remain. In the latest fiscal year, CIHR investments in the Proof of Principle program decreased significantly (Figure 19). This program requires matching contributions from industry, reflecting the difficulties Canadian industry is facing in acquiring venture capital for research and development, and the relatively low and decreasing "big pharma" R&D investment in Canada (Figure 20). To overcome these challenges, CIHR has appointed a Commercialization Advisory Committee, which includes experts in commercialization, technology transfer, the biotechnology and pharmaceutical industries, and company management. The committee has helped to redesign CIHR's commercialization funding opportunities to improve outcomes in these difficult economic times.
Figure 19: CIHR spending on commercialization programs
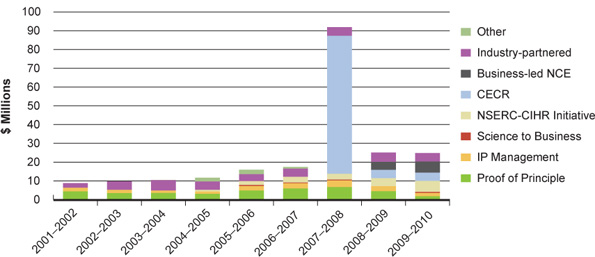
Figure 20A: Venture capital investments in the life sciences in Canada
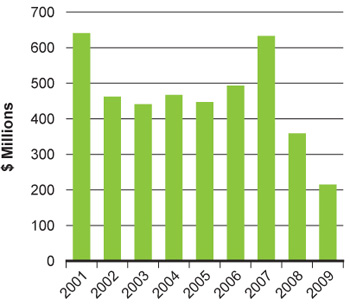
A: Data from Annual Statistical Reports of Canada's Venture Capital and Private Equity Association
Figure 20B: R&D to sales ratio, Canada and seven comparator countries
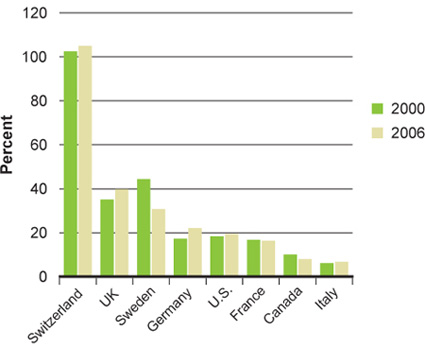
B: Amended from Figure 23, Patented Medicine Prices Review Board 2008 Annual Report
Ethics
"CIHR should increase its emphasis on research in ethics as well as its governance responsibilities that ensure that the research that it funds meets the highest ethical standards."
There have been major developments in both research on ethics, and ethical standards in health research, as advised by the IRP. The ethics function at CIHR consists of the Standing Committee on Ethics to advise GC, the Ethics Office to lead and coordinate CIHR's mandate in ethics and ethics designates on all Institute Advisory Boards to provide advice to institutes. The Ethics Office also supports GC's Stem Cell Oversight Committee, which reviews all research involving human pluripotent stem cells to determine its conformity to the CIHR Stem Cell Research Guidelines; and the Research Integrity Committee, which considers allegations of non-compliance with Tri-Council policies.
Important achievements of the Ethics Office include:
-
publishing Guidelines for Health Research Involving Aboriginal Peoples (2007), which informed Chapter 9 of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS)
-
providing major input into the TCPS 2nd Edition in areas of health research
-
influencing and contributing to the development of the Tri-Council process for addressing allegations of non-compliance with Tri-Council policies (2009)
-
developing Best Practices for Research Involving Children and Adolescents (fall 2010); and an ethical framework for Partnerships with the Private Sector (fall 2010)
Figure 21: Number and value of ethics grants
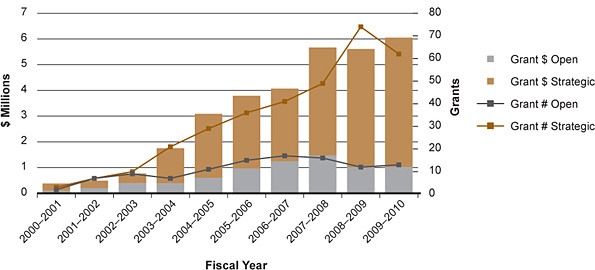
Through its strategic funding envelope of $2 million per year (which supplements the support available through CIHR's open funding competitions), the Ethics Office has been building capacity and supporting the existing research community. Figure 21 shows that CIHR now invests more than $6 million per year in 75 ethics-related grants. A further $1 million supports 33 training and salary awards.
A first Ethics strategic plan for 2010–2015 was developed in alignment with the CIHR Roadmap.
Evaluation
"End-of-grant reports provide an important mechanism in accumulating data on achievements that can be used for future evaluations. There are standard metrics in all (research) settings and more effort needs to be invested in ensuring that these are collected and analyzed to plot the relative success of the organization. This process needs to be addressed immediately so that information is available to assess CIHR objectively on its performance."
Resources and expertise for program evaluation have increased. CIHR has adopted a modified "payback" framework for capturing the value of investments in health research, similar to that proposed by the Canadian Academies of Health Sciences42 and focusing on advancing knowledge, research capacity building, informing decision making, health impacts and broad economic and social impacts. A five-year rolling evaluation plan is published each year.43 Programs evaluated to date include the Training Programs, Canada Graduate Scholarships and Networks of Centres of Excellence (the latter two in collaboration with the other granting agencies). Studies on the outcomes from Open Operating Grants and Salary programs are in progress. The institutes have evaluated many of their most important research initiatives and this information is found in their individual reports.
A Research Reporting System captures information on the outputs and outcomes from all CIHR-funded research within 18 months of the end of the funding period.
Communications
"Communication remains an important and challenging activity for the CIHR, particularly the range of potential audiences, including funding partners, provincial and federal governments, universities, health researchers, international agencies and the citizens of Canada. CIHR management needs to consider creative approaches to the utilization of a wide range of communication sources and resources including effective use of electronic and web-based dissemination, and should continue to improve its communication with key stakeholders."
Communications and Public Outreach targets the media through press releases, expert alerts, ministerial announcements, a monthly e-newsletter to journalists and short profiles about CIHR-funded researchers. There has been an eight-fold increase in the number of media mentions of CIHR since the 2006 IRP report: from 561 in 2005–2006 to 4,363 in 2009–2010. To raise CIHR's profile among journalists and promote excellence in Canadian health journalism, the CIHR Journalism Awards and journalist workshops were instituted. CIHR regularly communicates the results and impacts of CIHR investments in health research with Members of Parliament. Each fall, CIHR holds the Canadian Health Research Awards, recognizing health research excellence. This event receives widespread publicity. CIHR engages members of the public directly through Cafés Scientifiques – informal interactions between the public and experts on a health issue, held at a café, pub or restaurant. CIHR held 104 Cafés across the country in 2009.
To connect health researchers with youth, CIHR developed its Synapse program44 with the result that in 2008–2009, 5,300 CIHR mentors devoted 27,300 hours of their time to educate 112,800 Canadian youth about the merits of science and health research. CIHR's successful social media presence has expanded in the past year, with English and French Facebook pages45 that already have more than 40,000 fans. In December 2008, CIHR launched a fully redesigned website with client-centric information architecture, and a consistent presentation of the institutes, each of which publishes newsletters about research opportunities and achievements that are of special interest to its research and partner community.
Canada's research landscape
"A major outstanding challenge for the CIHR and health research in Canada is the apparent lack of co-ordination at the federal and provincial levels of the many different types and sources for funding for different aspects of health research. Support for infrastructure and research posts are welcome but must be aligned with the operating grants that are necessary to keep the research enterprise running."
Since the first International Review, all federal research agencies, including CIHR, have strengthened their collaboration. This collaboration operates at multiple levels, including joint programs, administrative structures and funding policies. Presidents of the Natural Sciences and Engineering Research Council, the Social Sciences and Humanities Research Council, CIHR, and the Canada Foundation for Innovation meet every three to six weeks to discuss issues of common concern, advance joint initiatives and explore strategic issues. Vice-presidents also meet on a regular basis to follow up on issues raised by the presidents, as well as to initiate and contribute to collaborative activities. An example of the outcomes of such meetings is the Institute of Population and Public Health-led International Research Initiative on Adaptation to Climate Change,46 which also involves the International Development Research Centre, and supports the formation of multinational teams from Canada and low- and middle-income countries.
In addition, CIHR has strengthened its ties with the National Research Council (NRC) and Genome Canada. In 2008, CIHR and Genome Canada initiated major national and international collaborations such as the Canada–California partnership on Cancer Stem Cells (see below) and, more recently, a partnered program called Advancing Technology Innovation through Discovery that links next-generation sequencing technologies with gene discovery projects on childhood diseases.
Despite this broadened dialogue, the complex balance of funding among highly qualified personnel, infrastructure and maintenance and operating support – an issue that largely lies with budgetary decisions by the federal government – has remained a challenge.
- Date modified: